Jennifer Forman Orth and Matt Pelikan

Habitat with dead canopy trees, the Woods Preserve (Nature Conservancy), West Tisbury, MA. Photograph by Matt Pelikan.
For many of us in the world of pest management, the invasive insect known as the gypsy moth (Lymantria dispar) had fallen off the radar in recent years, after having caused massive tree deaths in the 1980s and then going quiet. But since 2015, the gypsy moth has come back in a big way. In this article, we take a look at what impact this outbreak could have on birds, and how birds can impact gypsy moth populations in return.
Gypsy Moths: The Problem
Most Massachusetts residents are aware of the impact that gypsy moth caterpillars have had in our state over the past few years. If people were not familiar with the name gypsy moth, they surely knew those “big black caterpillars.” In 2015 and 2016, a large part of the state was subject to defoliation by this pest, including portions of Barnstable, Plymouth, and Bristol Counties, the eastern parts of Hampden and Hampshire Counties, southern Middlesex and Worcester Counties, and the northernmost part of Essex County (Figure 1). The gypsy moth is not new to our state—it was first introduced to North America in Somerville, Massachusetts, more than 120 years ago. However, for a couple of decades until the recent outbreak, we had been in a sort of uneasy détente with the pest. This period of calm was made possible by the flourishing of a fungus, Entomophaga maimaiga, starting in 1989 (Hajek 1996). E. maimaiga, known as an “entomopathogenic” fungus because it causes disease in insects, was one of many biological controls released in an effort to combat gypsy moth. It infects gypsy moths at the caterpillar stage, killing the caterpillars late in their life cycle. While this fungus won’t ever be able to eradicate gypsy moths, until recently it kept most infestations in check.
That all changed in early spring of 2015, when parts of the state began to experience drought conditions. By June 2015, the entire state was at a level of Abnormally Dry (D0) or higher according to the U.S. Drought Monitor’s Drought Intensity Scale, with more than half the state at a Moderately Dry level (D1), including Barnstable County and many of the adjacent towns and cities in Bristol and Plymouth County. Like many fungi, E. maimaiga needs moisture and humidity for its spores to germinate. Dry conditions meant the fungus couldn’t thrive, which was a boon for gypsy moth populations. Thousands upon thousands of caterpillars ate their way through the summer, pupated, and by July 2015 had emerged in large numbers as adult moths. These moths went on to produce huge numbers of egg masses that overwintered into 2016.
While there was some drought relief in the winter and spring of 2016, drought conditions returned later that spring throughout much of the state. By July and August 2016, several counties were experiencing Severe Drought (D2) or even Extreme Drought (D3) levels (National Drought Mitigation Center, 2016). The high number of egg masses combined with drought conditions that prevented the fungus from proliferating meant that an even bigger gypsy moth year was on deck. Indeed, aerial surveys by the Massachusetts Department of Conservation and Recreation (DCR) indicated that more than 350,000 acres in the state were defoliated by gypsy moth caterpillars in the summer of 2016 (Massachusetts DCR 2016), a 300,000-acre increase over 2015 and the biggest defoliation in decades (Figure 1).
The 2016 gypsy moth outbreak garnered a lot of attention. When the caterpillars reach their final instars, their large size and black, spiky appearance makes them quite noticeable. At outbreak levels, the caterpillars can frequently be seen migrating to nearby food sources by the hundreds. Defoliations are usually hyper-localized; even in heavily infested areas, one homeowner might see all of his broadleaf trees stripped of foliage, while the yard across the street has minimal tree damage. When the caterpillars pupated and adult moths emerged in July, the initial daytime flight of the males was so massive that it made the evening news (Hager 2016). These recent repeated defoliations have raised questions about possible impacts on the environment, including bird populations. In this article, we look more closely at the complex relationship between birds and gypsy moths. In particular, we examine how gypsy moths might influence the abundance of Massachusetts birds, what the impact on birds might be after repeated defoliations caused by gypsy moth outbreaks, and what impact birds may have on this introduced forest pest.
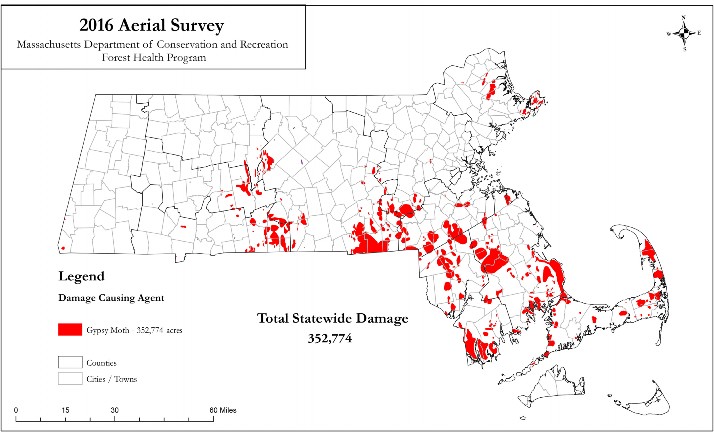
Fig. 1. 2016 Aerial Survey showing areas damaged by Gypsy Moths.
Gypsy Moth Outbreaks: Direct Impacts
The most obvious direct impact of a major gypsy moth infestation is the defoliation of trees and shrubs. Before we can delve into the effect defoliation can have on birds, we must first understand what it means for plants. Gypsy moth caterpillars will consume the leaves of more than 500 types of trees, shrubs, and vines, including broadleaf and coniferous species. Their preferred targets are oak, birch, poplar, willow, alder, basswood, and apple. They have also been observed eating maple, sassafras, hornbeam, elm, cherry, hickory, and many other common components of hardwood forests in Massachusetts. Older caterpillars (later instars) have been observed eating coniferous species such as pine, spruce, and hemlock, likely because all of their preferred food sources had already been consumed (McManus et al. 1979). As a result, in outbreak years, there are few woody plants in Massachusetts forests whose leaves won’t become gypsy moth caterpillar food.
A single defoliation, even if complete, is unlikely to kill a large, mature tree or shrub. A broadleaf tree, e.g., an oak or a maple, will typically respond to defoliation by putting out a new set of leaves within a few weeks. However, repeated defoliations over the course of several growing seasons can lead to dead branches and eventually dead trees, particularly when environmental conditions are already stressful, such as during a drought. Full defoliations of seedlings and small saplings can lead to plant death much more quickly.
In the eastern part of the state, gypsy moth damage has been occurring after another introduced species, winter moth (Operophtera brumata), has already caused significant defoliation to broadleaf trees such as maple, oak, and elm (Elkinton and Boettner 2016). Since winter moth caterpillars feed from April to early May, trees and shrubs are often just putting out a new set of leaves when gypsy moth caterpillars begin feeding in May and June. That means that the same plants could end up trying to put out a total of three sets of leaves each season. This kind of resource expenditure jeopardizes future growth and the ability of the tree or shrub to survive. Gypsy moth caterpillars that hatch in a part of the state where winter moth has already defoliated the majority of broadleaf trees will also move on to less preferred host trees, such as pine and spruce. Defoliation of these conifers can have more severe and immediate consequences, since conifers do not put out a new set of leaves each year, and producing new needles is an extremely costly use of the tree’s resources.
Gypsy Moth Defoliation: Impacts on Birds
What impact might the defoliation caused by gypsy moths have on birds? The first most obvious point is that defoliation removes shelter, reducing the number of suitable nesting sites for ground- and tree-nesting birds, including many of our songbirds. Defoliation also potentially reveals nesting sites to predators and may cause nesting birds to abandon their nests. Unfortunately, peak defoliation during a gypsy moth infestation occurs in July, which typically coincides with critical points in the nesting season for local birds (Thurber et al. 1994). Nest exposure when young birds are readying to fledge means they become visible to predators at a time when they are in some respects most vulnerable.
In addition, exposure of the forest floor to sunlight following defoliation of the upper canopy and the shrub layer, typical of a severe gypsy moth outbreak, can lead to indirect impacts on forest fauna. The lack of foliage means less shade, causing the forest floor to experience higher daytime temperatures and a lack of humidity that ground-nesting birds may find inhospitable (Smith and Lautenschlager 1981). The opening up of the shrub layer may also encourage predators to move into the area if they perceive prey is accessible (Smith and Lautenschlager 1981). Birds that forage in the leaves of live trees and shrubs may also encounter a lack of foraging sites and could experience food shortages later in the season because little food is left to support native caterpillars.

Late-instar female gypsy moth caterpillar. Photograph by Jennifer Forman Orth.
All of these factors imply that, in the short term, the forest environment can become inhospitable for breeding birds following a gypsy moth outbreak. Various studies have attempted to measure this impact. Smith and Lautenschlager (1981) noted that in infested areas where refoliation does occur, the birds and mammals will flee but will return to the area within two to three months. In an experiment done with artificial nests (Thurber, McClain, and Whitmore 1994), nest predation was higher in defoliated sites, and predation was also more frequent for ground nests than for nests placed more than one meter off the ground. Gypsy moth infestations are also associated with decreases in bird species associated with closed canopy forests (Gale, DeCecco, McClain, Marshall, and Cooper 2001), which makes sense because defoliation dramatically alters the makeup of the canopy.
Over several years, repeated defoliation of the upper canopy of the forest can lead to tree death. But these dead trees may be beneficial for some bird species. For example, Eastern Towhee is a ground- and shrub-nesting species that forages in the forest understory and thrives in early successional habitat. Bell and Whitmore (1997) found that populations of Eastern Towhee increased following a gypsy moth outbreak, because the defoliation opened up the canopy, and the sunlight exposure led to the creation of a denser shrub layer. Thurber, McClain, and Whitmore (1994) noted that the growth of the shrub understory following defoliation of the upper canopy should bring in additional birds, but that this increase in bird numbers would also attract mammalian predators, suggesting that the net impact on birds might be neutral. These authors also predicted that birds nesting in the mid to upper canopy, such as Scarlet Tanager, Eastern Wood-Pewee, and Wood Thrush, would experience increased predation following defoliation. However, Bell and Whitmore (2000) found that for shrub and sub-canopy nesters, e.g., Indigo Bunting and Wood Thrush, the creation of additional shrub habitat offset any negative impacts due to predation.
Snags—standing deadwood—resulting from tree death can also become nesting locations for some species. Showalter and Whitmore (2002) found that overall abundance of cavity-nesting birds increased for the first five years following a gypsy moth outbreak, though that abundance then decreased over the following six years. In that study, primary cavity nesters such as Red-bellied Woodpecker, Pileated Woodpecker, and Northern Flicker were found to have a positive association with snags, as did secondary cavity nesters such as Black-capped Chickadee, and these species were found to take advantage of new nesting habitat created by snags immediately following an outbreak. However, populations of other primary cavity nesters, including Downy Woodpecker, Hairy Woodpecker, Tufted Titmouse, and White-breasted Nuthatch, did not increase when the number of snags increased, leading the authors to surmise that other factors suppress their populations.
There has also been some discussion if creation of snags following several years of defoliation might negatively impact ground-nesting birds by leading to increased nest predation or parasitization by birds that use the snags as perches to spot prey. However, research has not demonstrated a significant impact, with one study showing no increase in raptor predation or nest parasitization (Bell and Whitmore 1997), and a separate study showing that the creation of larger snags did not lead to an increase in nest parasitization by cowbirds (Bell and Whitmore 2000). In both studies, the authors suggested that the buildup of the shrub canopy following defoliation of the upper canopy limited opportunities for predation and parasitization by concealing any nests that were present.
Gypsy Moths as a Food Source
Gypsy moths start out their lives inside light brown egg masses that are laid by the female moths, mainly on tree trunks and branches but sometimes on outdoor furniture and other structures. The egg masses are a combination of eggs and hairs from the body of the female moth. The caterpillars hatch from the eggs in early to mid-May, and at their earliest life stages—the first few instars—can be found hiding on the undersides of mostly intact leaves. During this time, the hairs covering the caterpillars are smaller and thinner than the robust bristles found on later instars, when the caterpillars are larger and closer to pupation. The caterpillars pupate in late June or early July, forming dark brownish-red pupal cases that can often be found in clusters on tree trunks, fences, or the sides of buildings. The adult moths emerge from the pupal cases after about two weeks. The male moths are grey, and it is these males that you will see in flight during big outbreak years, or attracted to lights at night. The females are white and much larger than males, and though they have wings, they cannot fly. Instead, they usually flutter around the area where they emerged from their pupae, until a male arrives to mate.
Birds have varying interests in gypsy moths as a food source, depending on the bird species in question and the life stage of the insect. Table 1 includes a list of 41 Massachusetts bird species documented as having eaten one or more life stages of the gypsy moth. Only five species have been documented eating egg masses, presumably because the masses are covered with hairs from the body of the female, rendering them distasteful or difficult to eat (Leonard 1981). Nonetheless, Forbush and Fernald (1896) noted that Black-capped Chickadees and House Sparrows eat the eggs, and White-breasted Nuthatches have been observed picking at the egg masses to get at other insects underneath. In contrast, in areas of Europe where gypsy moth is native, several bird species are known to eat the egg masses, as reported by Campbell (1981). As with the egg masses, the pupae are seldom eaten by birds in the eastern United States, though Hairy Woodpeckers, Eastern Wood-Pewees, cuckoos, vireos, and other species have been observed doing so (Smith 1985).
Most observations about the consumption of gypsy moths by birds come from the caterpillar life stage (larvae). The majority of this information comes from gut studies done by Forbush and Fernald (1896). During outbreak years, they found that significant percentages of the gut content of several bird species consisted of gypsy moth caterpillars. However, not many bird species have adapted to eat hairy caterpillars such as those of the gypsy moth. A study done by Whelan, Holmes, and Smith (1989) found that North American bird species generally prefer nonhairy caterpillars, and if offered both gypsy moth caterpillars and a nonhairy species in a feeding experiment, they will preferentially choose the nonhairy species. That study also found that birds were more willing to accept earlier gypsy moth instars, likely because the hairs were less distasteful or obtrusive to the birds. Leonard (1981) also noted that many more bird species will consume early instar gypsy moth larvae. In contrast, Smith and Lautenschlager (1981) investigated the gut contents of 17 different bird species and found that the majority contained mainly late-instar larvae, even though sampling was done in both June and July. It is difficult to know, though, if Smith’s results reflect a preference that birds have for mature gypsy moth caterpillars or a shortage of preferable food.

Black-billed Cuckoos are caterpillar specialists. Photograph ©Shawn P. Carey.
Notable among our woodland birds as predators of gypsy moth caterpillars are the cuckoos. Yellow-billed and Black-billed cuckoos are caterpillar specialists, eating both hairy and spiny caterpillars. They can eat these caterpillars specifically because they have evolved the fascinating ability to regrow their stomach linings. Once the cuckoo’s stomach lining is completely clogged with hairs and spines from the caterpillars it has digested, the bird regurgitates the used stomach lining in a pellet form, removing all the spines and hairs along with it (Forbush 1907, cited in Bent 1940). Both cuckoo species, along with grackles and Red-winged Blackbirds, are attracted to gypsy moth infestations and will enter new territory once the caterpillars reach outbreak levels (Leonard 1981). Once these birds arrive, they consume large numbers of the caterpillars. Other opportunistic species known to be attracted to gypsy moth caterpillar infestations include crows, Chipping Sparrows, starlings, and cowbirds (Smith and Lautenschlager 1981), though Smith (1985) noted that the Chipping Sparrows he captured had no gypsy moth caterpillars in their guts. There is also a second suite of birds that, rather than arriving only when outbreak levels are high, instead eat gypsy moth caterpillars as a regular part of their diet, presumably helping to keep the pests at low levels until an outbreak occurs. This group includes Black-capped Chickadee, Blue Jay, Eastern Towhee, Baltimore Oriole, and Gray Catbird (Smith and Lautenschlager 1981), all of which are relatively common species that forage in a wide variety of habitats and often produce at least two broods per season.
A few bird species have also been documented eating adult gypsy moths. Blue Jays, for example, have been observed vigorously feeding on adult male moths during outbreak years. Specifically, Blue Jays on Cape Cod were seen congregating on tree trunks and lower branches right after sunrise, targeting the resting area of the moths. As the day went on and the moths left the tree trunks to hide in nearby shrub foliage, the Blue Jays were observed foraging in the shrubs, pulling the male moths from the undersides of leaves, even flying repeatedly straight into the shrubs, flapping their wings in order to scare up and dislodge the hiding moths (Odell 1977 cited in Smith and Lautenschlager 1981). Other bird species that feed on adult gypsy moths include Indigo Bunting, Ovenbird, Common Yellowthroat, and Eastern Phoebe. Nonetheless, the adult moths are not known to be a significant part of the diet of any bird species.
Direct Impacts of Gypsy Moth Infestations on Birds
It makes sense that the caterpillar life stage of gypsy moth is the most important to birds, because caterpillars are active when birds are nesting, and fledglings are frequently fed caterpillars for their high protein content (Smith and Lautenschlager 1981). Gale, DeCecco, McClain, Marshall, and Cooper (2001) found that short-term impacts of a gypsy moth caterpillar influx on bird abundance were numerous. For example, Black- and Yellow-billed cuckoos, noted caterpillar specialists, increased in local abundance two years before the gypsy moth hit outbreak levels and then were gone as soon as the outbreak abated. Indigo Buntings had a similar increase right before an outbreak but took about five years to get back down to typical population levels.
| Family |
Common Name |
Scientific Name |
Life Stages of Gypsy Moth
Consumed |
| Cuculidae |
Yellow-billed Cuckoo |
Coccyzus americanus |
L, P |
| |
Black-billed Cuckoo |
Coccyzus erythropthalmus |
L, P |
| |
|
|
|
| Picidae |
Downy Woodpecker |
Picoides pubescens |
E, L |
| |
Hairy Woodpecker |
Picoides villosus |
L, P |
| |
Northern Flicker |
Colaptes auratus |
L |
| |
|
|
|
| Tyrannidae |
Eastern Wood-Pewee |
Contopus virens |
L, P, A |
| |
Least Flycatcher |
Empidonax minimus |
L, A |
| |
Eastern Phoebe |
Sayornis phoebe |
P, A |
| |
Great Crested Flycatcher |
Myiarchus crinitus |
P, A |
| |
Eastern Kingbird |
Tyrannus tyrannus |
P, A |
| |
|
|
|
| Vireonidae |
White-eyed Vireo |
Vireo griseus |
L |
| |
Yellow-throated Vireo |
Vireo flavifrons |
L, P, A |
| |
Red-eyed Vireo |
Vireo olivaceus |
L, P, A |
| |
|
|
|
| Corvidae |
Blue Jay |
Cyanocitta cristata |
E, L, P, A |
| |
American Crow |
Corvus brachyrhynchos |
L, P, A |
| |
|
|
|
| Paridae |
Black-capped Chickadee |
Poecile atricapillus |
E, L, P, A |
| |
|
|
|
| Sittidae |
White-breasted Nuthatch |
Sitta carolinensis |
E |
| |
|
|
|
| Troglodytidae |
House Wren |
Troglodytes aedon |
L |
| |
|
|
|
| Turdidae |
Eastern Bluebird |
Sialia sialis |
L, P, A |
| |
Wood Thrush |
Hylocichla mustelina |
L |
| |
American Robin |
Turdus migratorius |
L, P, A |
| |
|
|
|
| Mimidae |
Gray Catbird |
Dumetella carolinensis |
L, P, A |
| |
Brown Thrasher |
Toxostoma rufum |
L, P, A |
| |
|
|
|
| Sturnidae |
European Starling |
Sturnus vulgaris |
L |
| |
|
|
|
| Passeridae |
House Sparrow |
Passer domesticus |
E, L, P, A |
| |
|
|
|
| Parulidae |
Ovenbird |
Seiurus aurocapilla |
L, A |
| |
Black-and-white Warbler |
Mniotilta varia |
L, A |
| |
Common Yellowthroat |
Geothlypis trichas |
L, A |
| |
American Redstart |
Setophaga ruticilla |
L, A |
| |
Yellow Warbler |
Setophaga petechia |
L, P, A |
| |
Chestnut-sided Warbler |
Setophaga pensylvanica |
L, A |
| |
Black-throated Green Warbler |
Setophaga virens |
L, A |
| |
|
|
|
| Emberizidae |
Eastern Towhee |
Pipilo erythrophthalmus |
L, P, A |
| |
Chipping Sparrow |
Spizella passerina |
L, A |
| |
|
|
|
| Cardinalidae |
Scarlet Tanager |
Piranga olivacea |
L, P, A |
| |
Rose-breasted Grosbeak |
Pheucticus ludovicianus |
L |
| |
Indigo Bunting |
Passerina cyanea |
A |
| |
|
|
|
| Icteridae |
Red-winged Blackbird |
Agelaius phoeniceus |
L |
| |
Common Grackle |
Quiscalus quiscula |
L |
| |
Brown-headed Cowbird |
Molothrus ater |
L |
| |
Baltimore Oriole |
Icterus galbula |
L, P, A |
| Legend: E = eggs, L = Larvae, P = Pupae, A= Adults |
Table 1. Massachusetts bird species that are known to eat gypsy moths. Sources: Forbush and Fernald (1896), McManus et al. (1979), Smith and Lautenschlager (1981).
In contrast to years where gypsy moths are present but not at outbreak levels, or are rising in numbers but in extremely limited geographical areas, years of extensive infestation can result in food pulses being injected into the forest ecosystem. One study looked at the impact of these repeated pulses on bird abundance over three decades in Connecticut, Pennsylvania, and Virginia, and found an overall increase in populations of all woodpecker species over time, suggesting that the woodpeckers have learned to take advantage of outbreak years (Koenig, Walters, and Liebhold 2011). Research has found few other long-term trends, because the impacts of the moths are too variable. Koenig’s study also found that, over shorter time periods, Red-headed Woodpecker and Northern Flicker numbers increased during the breeding season in outbreak years, whereas Downy Woodpecker populations decreased. Though some bird species tie their breeding cycle to times when caterpillars are most abundant (Hinks et al. 2015), we have no evidence that such synchronization occurs specifically with gypsy moth outbreaks, perhaps because these outbreaks generally occur several years apart and last for only one to three years. Taken together, these studies suggest either that short-term trends are not good predictors of long-term bird populations, or that the long-term woodpecker success found by Koenig was related to factors other than gypsy moth outbreaks. Gale DeCecco, McClain, Marshall, and Cooper (2001) concluded that impacts of gypsy moth on birds will always be short-term, provided that there is little tree mortality.

Gypsy Moths, Framingham. Photograph by Jennifer Forman Orth.
Bird Impacts on Gypsy Moths
Much of the older research on birds and gypsy moths has focused on if birds could reduce populations of this pest, thus alleviating an outbreak. Campbell (1977, in Campbell 1981) found that excluding birds and small mammals from experimental plots had a stronger impact on gypsy moth populations than excluding mammals alone. Smith and Lautenschlager (1981) also noted that migrating warblers sometimes pass through forests when gypsy moth caterpillars are young and could make a dent in populations in areas where they stop to rest.
Gypsy moth caterpillars are generalists, feeding on many different plants. The “enemy-free space cascade hypothesis” suggests that, unlike host-specific caterpillars, caterpillars that use many host plants will in turn have a variety of predators feeding on them, and that this predator pressure from birds will create a strong trophic cascade down the food chain that leads to a significant decrease in herbivory (Singer et al. 2014). However, once an outbreak occurs, predation by birds and other predators has been found to be insufficient to affect gypsy moth populations (Smith and Lautenschlager 1981). As much as defoliation impacts the forest in the short term, the cyclical nature of gypsy moth outbreaks, in combination with the fact that gypsy moths are important food sources for only a few predators (Smith 1985), means that the overall effect of birds on gypsy moths is limited.
That conclusion has not, however, kept some from suggesting natural “solutions” involving birds as a way of combating the gypsy moth problem. In Eurasia, nesting boxes are frequently placed in outbreak areas to encourage cavity-nesting birds to settle in the area, with the hope that the birds will consume caterpillars (Leonard 1981, Smith and Lautenschlager 1981). Leonard (1981) also recommends retaining brush in the forest understory as a way to promote populations of ground- and shrub-nesting species. Others have suggested that foresters should plant and encourage tree species that would provide the most habitat and shelter for bird species known to eat gypsy moth (Smith and Lautenschlager 1981). Unfortunately, because current research indicates that birds do not control gypsy moth populations in outbreak years, it is unlikely that such strategies will lead to control of gypsy moth outbreaks. Perhaps that is because there is already enough food available for birds to thrive without consuming gypsy moths, or because so few bird species actually prefer the caterpillars of gypsy moths if other more palatable caterpillars are available.
Gypsy Moth in Massachusetts: What Does the Future Hold?
There are now parts of Cape Cod, Bristol, Plymouth, and Worcester Counties that have experienced at least two consecutive years of high gypsy moth infestations and severe tree defoliation. The volume of egg masses on the trees in outbreak areas indicate that 2016 was once again a very successful reproductive year for gypsy moth. The Massachusetts DCR is currently predicting a third year of significant defoliations in 2017. Even though early spring storms in 2017 have alleviated drought conditions across most of Massachusetts, the two preceding years of dry conditions mean that there will likely not be enough Entomophaga maimaiga spores around this summer to truly knock down gypsy moth caterpillar populations (Massachusetts DCR 2017).

Adult male gypsy moth. Photograph by Jennifer Forman Orth.
What would another year of defoliation mean for the future of forests in Massachusetts? As discussed above, long-term impacts of gypsy moth infestations on forest ecology are typically limited because the outbreaks do not last longer than one to three years, thus restricting the number of tree deaths and allowing bird populations to quickly recover or to decline following any bumps in abundance. But some parts of the state are now heading into what could be their third or even fourth year of high gypsy moth levels. If drought conditions return in the summer of 2017 or in subsequent years, the repeated defoliations and associated tree deaths that occur could lead to this pest setting back the process of succession in some forests (Bell and Whitmore 1997). The drought itself will only compound the problem, because lack of water can hasten death in a tree or shrub already damaged by defoliation or disease. Under such stresses, hardwood forest ecosystems may be altered, as selective pressure will favor species that gypsy moth does not like to eat (Twery 1991). These species would include trees such as ash, butternut, walnut, dogwood, tulip poplar, and catalpa, as well as shrubs such as American holly, mountain laurel, and rhododendron (McManus et al. 1979). Drought-resistant plants may also gain a competitive advantage.
It is difficult to predict how bird populations may be affected in the areas of Massachusetts most impacted by gypsy moth. Will the pine barrens in southeastern Massachusetts be reduced to nothing but low, scrubby shrubs, grasses, and herbs, with the canopy hospitable only to cavity-nesting birds that can take advantage of all the snags? Will the repeated exposure of the floors of oak and hickory forests to sunlight, combined with the death of nearby trees, create opportunities for invasive shrubs and small trees such as buckthorn, burning bush, barberry, and shrub honeysuckle? If so, there might be increased habitat for ground- and shrub-nesting bird species. On the other hand, forests might be unable to regenerate a closed canopy, with the open habitat remaining inhospitable to Scarlet Tanager, Eastern Wood-Pewee, and the warbler species that nest high in trees.
This review has examined the complicated relationship that has developed in Massachusetts among birds, the woodlands they inhabit, and the gypsy moth, an introduced pest that has festered in our state for over a century. Moth outbreaks, especially in the presence of other stresses such as drought, can substantially alter forest structure, and therefore avian habitat, in the shortterm and may also potentially have some impact over longer periods of time. Some bird species will benefit from these alterations, and others will lose out. The fact that these interactions are happening within the context of a changing climate adds further complexity, as does the potential introduction of other forest pests, pathogens, and invasive plants. With a large and skilled birding community and a long history of documented research into the state’s avian populations, Massachusetts is well positioned to support future studies to examine this issue from a wider perspective, with the hope that we can identify issues that put birds at risk and work toward alleviating them.
References
- Bell, J.L. and R.C. Whitmore. 1997. Eastern Towhee Numbers Increase Following Defoliation by Gypsy Moths. The Auk. 114 (4): 708-16.
- Bell, J.L. and R.C. Whitmore. 2000. Bird Nesting Ecology in a Forest Defoliated by Gypsy Moths. The Wilson Bulletin. 112 (4): 524-31.
- Bent, A.C. 1940. Life Histories of North American Cuckoos, Goatsuckers, Hummingbirds and Their Allies: Orders Psittaciformes, Cuculiformes, Trogoniformes, Coraciiformes, Caprimulgiformes and Micropodiiformes. Washington: U.S. Government Printing Office.
- Campbell, R.W. 1981. Population Dynamics - Historical Review (Chapter 4). in The Gypsy Moth: Research Toward Integrated Pest Management. Technical Bulletin 1584: pages 65-86. C.C. Doane and M.L. McManus, editors. Washington: U.S. Forest Service, Science and Education Agency, Animal and Plant Health Inspection Service.
- Elkinton, J. and J. Boettner. 2016. Gypsy Moth Outbreak of 2016. Massachusetts Wildlife Magazine. #3. Accessed April 14, 2017.
- Forbush, E.H. and C.H. Fernald. 1896. The Gypsy Moth. Porthetria dispar (Linn.). Boston: Wright & Potter. Accessed April 14, 2017.
- Gale, G.A., J.A. DeCecco, M.R. Marshall, W.R. McClain, and R.J. Cooper. 2001. Effects of Gypsy Moth Defoliation on Forest Birds: An Assessment using Breeding Bird Census Data. Journal of Field Ornithology. 72 (2): 291-304.
- Hager, C. Moth Swarm Interferes With Plane At Logan Airport. July 6, 2016. CBS News Boston. Accessed April 3, 2017.
- Hajek, A.E. 1996. Entomophaga maimaiga: A Fungal Pathogen of Gypsy Moth in the Limelight. Proceedings of the Cornell Community Conference on Biological Control. 42: 1-2.
- Hinks, A.E., E.F. Cole, K.J. Daniels, T.A. Wilkin, S. Nakagawa, and B.C. Sheldon. 2015. Scale-Dependent Phenological Synchrony between Songbirds and Their Caterpillar Food Source. The American Naturalist. 186 (1): 84-97.
- Koenig, W.D., E.L. Walters, and A.M. Liebhold. 2011. Effects of Gypsy Moth Outbreaks on North American Woodpeckers. The Condor. 113 (2): 352-61.
- Leonard, D.E. 1981. Bioecology of the Gypsy Moth (Chapter 2). In The Gypsy Moth: Research Toward Integrated Pest Management. Issue 1584: pages 9-29. C.C. Doane and M.L. McManus, editors. Washington: U.S. Forest Service, Science and Education Agency, Animal and Plant Health Inspection Service.
- Massachusetts Department of Conservation and Recreation. State Environmental Officials Predict Another Year of Defoliation from Gypsy Moth in 2017. January 1, 2017. Massachusetts Department of Conservation and Recreation. Accessed April 3, 2017.
- Massachusetts Department of Conservation and Recreation. Forest Health: Massachusetts Forest Pest Aerial Survey Maps. 2015-16. Massachusetts Department of Conservation and Recreation. Accessed April 2, 2017.
- McManus, M.L., D.R. Houston, and W.E. Wallner. 1979. Gypsy Moth Handbook: The Homeowner and the Gypsy Moth: Guidelines for Control. Washington: U.S. Department of Agriculture, Combined Forest Pest Research and Development Program, Home and Garden Bulletin.
- National Drought Mitigation Center. 2016. United States Drought Monitor - Massachusetts Tabular Data Archive. Accessed April 14, 2017.
- Showalter, C.R. and R.C. Whitmore. 2002. The Effect of Gypsy Moth Defoliation on Cavity-Nesting Bird Communities. Forest Science. 48 (2): 273-81.
- Singer, M.S., I.H. Lichter-Marck, T.E. Farkas, E. Aaron, K.D. Whitney, and K.A. Mooney. 2014. Herbivore diet breadth mediates the cascading effects of carnivores in food webs. Proceedings of the National Academy of Sciences. 111 (26): 9521-26.
- Smith, H.R. 1985. Wildlife and the Gypsy Moth. Wildlife Society Bulletin. 13 (2): 166-74.
- Smith, H.R. and R.A. Lautenschlager. 1981. Population Dynamics - Gypsy Moth Predators (Chapter 4). in The Gypsy Moth: Research Toward Integrated Pest Management. Issue 1584: 96-125. C.C. Doane and M.L. McManus, editors. Washington: U.S. Forest Service, Science and Education Agency, Animal and Plant Health Inspection Service.
- Thurber, D.K., W.R. McClain, and R.C. Whitmore. 1994. Indirect Effects of Gypsy Moth Defoliation on Nest Predation. The Journal of Wildlife Management. 58 (3): 493-500.
- Twery, M.J. 1991. Effects of Defoliation by Gypsy Moth. Proceedings of the U.S. Department of Agriculture Interagency Gypsy Moth Research Review. pages 27-39. Gottschalk, K.W., M.J. Twery and S.I. Smith, editors. Radnor, PA: U.S. Forest Service General Technical Report NE-146.
- Whelan, C.J., R.T. Holmes, and H.R. Smith. 1989. Bird Predation on Gypsy Moth (Lepidoptera: Lymantriidae) Larvae: An Aviary Study. Environmental Entomology. 18 (1): 43-5.
Jennifer Forman Orth is an Environmental Biologist at the Massachusetts Department of Agricultural Resources, where she works on insect and plant pest issues. An avid macro photographer, she typically spends the wee hours stalking insects with a camera and flashlight.
Matt Pelikan lives on Martha’s Vineyard and is the conservation measures manager for the Massachusetts chapter of The Nature Conservancy. A former editor of Bird Observer, he is an enthusiastic observer of wildlife of all kinds.